lewis dot structure for hcl|Lewis Dot Structure for HCl : Pilipinas Hul 23, 2022 — A step-by-step explanation of how to draw the HCl Lewis Dot Structure (Hydrogen chloride). For the HCl structure use the periodic table to find the total number of valence electrons for. Hot Pinoy m2m: Masarap mag jakol - Blogger . Sarap nito
lewis dot structure for hcl,Learn how to draw the Lewis structure of HCl, a polar covalent diatomic molecule with a single bond and no hybridization. Find out the molecular geometry, electron geometry, polarity and FAQs of HCl.Hul 23, 2022 — A step-by-step explanation of how to draw the HCl Lewis Dot Structure (Hydrogen chloride). For the HCl structure use the periodic table to find the total number of valence electrons for.
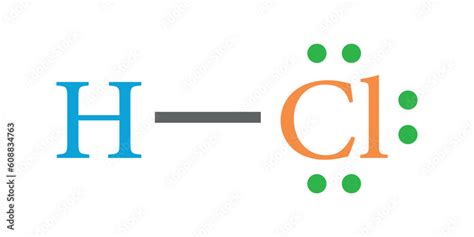
Lewis Structure for HCl (Hydrochloric Acid) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it might .Mar 10, 2021 — A step-by-step explanation of how to draw the correct Lewis Dot Structure for Hydrogen chloride (HCl gas).For the Hydrogen chloride structure use the periodi.May 22, 2023 — Learn how to draw the lewis dot structure of HCl (hydrogen chloride) with 6 simple steps and images. Check the valence electrons, octet rule, formal charge and stability of the .Lewis structure of Hydrogen chloride (HCl) contains only one H-Cl bond. There are no charges on atoms in HCl lewis structure because HCl is a neutral molecule. There is three lone pairs .By sharing valence electrons (each atom donates one), the stable \(\ce{HCl}\) molecule is formed. We will use a simplified representation of covalent bonds known as Lewis structures. These .
Ene 23, 2023 — Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in the molecule. .
The Lewis Structure for HCl (hydrochloric acid) is one of the easier dot structures to draw. When you draw the structure remember that Hydrogen (H) only needs two valence electrons to have .The Lewis Structure for HCl (hydrochloric acid) is one of the easier dot structures to draw. When you draw the structure remember that Hydrogen (H) only needs two valence electrons to have a full outer shell. Watch the video of Dr. B. drawing the dot structure for hydrochloric acid (HCl) and answer the questions below.
Hun 22, 2023 — Steps of drawing HCl lewis structure Step 1: Find the total valence electrons in HCl molecule. In order to find the total valence electrons in HCl (hydrogen chloride) molecule, first of all you should know the valence .

Ene 30, 2023 — Draw the Lewis dot structure of a given molecule or ion. Draw resonance structures of some molecules. Assign formal charge to an atom in a dot structure. Assess the stability of a structure by considering formal charges of atoms. Give examples for molecules and ions that do not follow the octet rule.Ene 30, 2023 — Draw the Lewis dot structure of a given molecule or ion. Draw resonance structures of some molecules. Assign formal charge to an atom in a dot structure. Assess the stability of a structure by considering formal charges of atoms. Give examples for molecules and ions that do not follow the octet rule.Hydrogen Chloride (HCl) Lewis Structure. Lewis structure of Hydrogen chloride (HCl) contains only one H-Cl bond. There are no charges on atoms in HCl lewis structure because HCl is a neutral molecule. There is three lone pairs on chlorine atom in .
Okt 20, 2020 — A Lewis structure contains symbols for the elements in a molecule, connected by lines and surrounded by pairs of dots. For example, here is the Lewis structure for water, H 2 O. Each symbol represents the nucleus and the core electrons of the atom.
We will use a simplified representation of covalent bonds known as Lewis structures. These drawings are also know by various other names, including Lewis dot structures or electron dot structures. Each dot in the structure represents one valence electron in the compound. For example, \(\ce{H_2}\) could be drawn as \(\ce{H} : \ce{H}\).May 22, 2023 — Lewis structure of HCl (or Hydrogen chloride) contains one single bond between the Hydrogen (H) and Chlorine (Cl) atom. The Chlorine atom has 3 lone pairs. Let’s draw and understand this lewis dot structure step by step. (Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely .Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.Dis 16, 2021 — Examples: Here we will take CO 2 molecule as an example to explain the procedure step by step:. 1. Total number of valence electrons: 4 (C atom) + 2×6 (2 O atoms) = 16. Always DOUBLE CHECK: In the correct Lewis structure, the total number of electrons involved (bonding plus non-bonding electrons) must be equal to this number, less or more are both .

For HCl, the Lewis dot diagram illustrates the transfer of an electron from hydrogen to chlorine, resulting in the formation of a polar covalent bond. What is a Lewis Dot Diagram? A Lewis dot diagram, also known as a Lewis structure, is a visual representation of the valence electrons in an atom or molecule.
Lewis Dot Structure for HCl Hun 27, 2022 — A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in .lewis dot structure for hclWe can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen .Lewis Symbols. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure \(\PageIndex{1}\) shows the Lewis symbols for the elements of the third period of the periodic table.
Since the overall formal charge is zero, the above Lewis structure of Cl 2 is most appropriate, reliable, and stable in nature.. Molecular Geometry of Cl 2. Cl 2 has a linear electron geometry. This is due to the fact that all diatomic molecules or any molecule with only two atoms will have a linear geometry or shape as these molecules contain two atoms that are connected with a .Write the Lewis structures for carbon tetrachloride and phosgene. Answer. PROBLEM \(\PageIndex{7}\) The arrangement of atoms in several biologically important molecules is given here. Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs. Do not add any more atoms.Abr 11, 2022 — This will lead to the generation of partial positive charge and partial negative charge on hydrochloric acid Lewis dot structure. Due to the differences in the electronegativities, hydrochloric acid will become a polar covalent molecule with a dipole moment of 1.03 D. . HCl gas-only has molecules and hydrochloric acid has ions H + and Cl .
lewis dot structure for hcl Lewis Dot Structure for HCl Hun 20, 2023 — Example \(\PageIndex{1}\): Lewis Structures. Solution; Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom.The kernel of the atom, i.e., the nucleus together with the inner electrons, is represented by the chemical symbol, and only the valence electrons are .
lewis dot structure for hcl|Lewis Dot Structure for HCl
PH0 · Lewis structure of HCl
PH1 · Lewis Structures
PH2 · Lewis Structure of HCl (With 6 Simple Steps to Draw!)
PH3 · Lewis Structure of HCl
PH4 · Lewis Structure for HCl (Hydrochloric Acid)
PH5 · Lewis Dot Structure for HCl
PH6 · Hydrogen Chloride (HCl) Lewis Structure
PH7 · How to Draw the Lewis Dot Structure for Hydrogen chloride
PH8 · How to Draw the Lewis Dot Structure for HCl: Hydrogen chloride
PH9 · Drawing the Lewis Structure for HCl (Hydrochloric Acid)
PH10 · Draw the Lewis Structure of HCl (hydrogen chloride)
PH11 · 4.1: Lewis Electron Dot Structures